Water Chemistry
Water is essential to life. It is the most common mineral on the earth’s surface and the most sought for. It is the major component of all living matter accounting for almost 80% of its composition.
In its pure state, it is a colorless, odorless and tasteless liquid. Being a good solvent, it dissolves many substances to varying degrees. For this reason, several impurities are found in the natural sources of water.
Barely 0.1% of the total volume of water on the earth is available as fresh water. The consumption of water for domestic, industrial and agriculture totals about 250 m3 per person per year with a great disparity between the developing countries and the advances nations. This precious and scarce resource is further threatened by mindless development and pollution. It is imperative that water be protected, used carefully and recycled to preserve this vital and dwindling resource.
Water as naturally available must be treated for general consumption or for specific industrial uses.
Why is Wastewater Treatment essential?
Sewage treatment, also known as wastewater treatment, is the process of purifying water to make it fit for reuse or release back into the environment by eliminating part or all of the impurities.
Effective wastewater treatment is crucial because untreated wastewater typically contains high concentrations of organic material, a variety of pathogenic microorganisms, nutrients, and toxic compounds that can be detrimental to human health, the environment, and waterways.
Both residential and commercial wastewater treatment are changing. In the past, its purpose was to filter wastewater before it could be safely released into the surrounding area. Wastewater is now recognized as a useful resource that can be used to produce energy, nutrients, and water for industrial, agricultural, and even drinking uses.
Waste water treatment is essential due to following reasons:
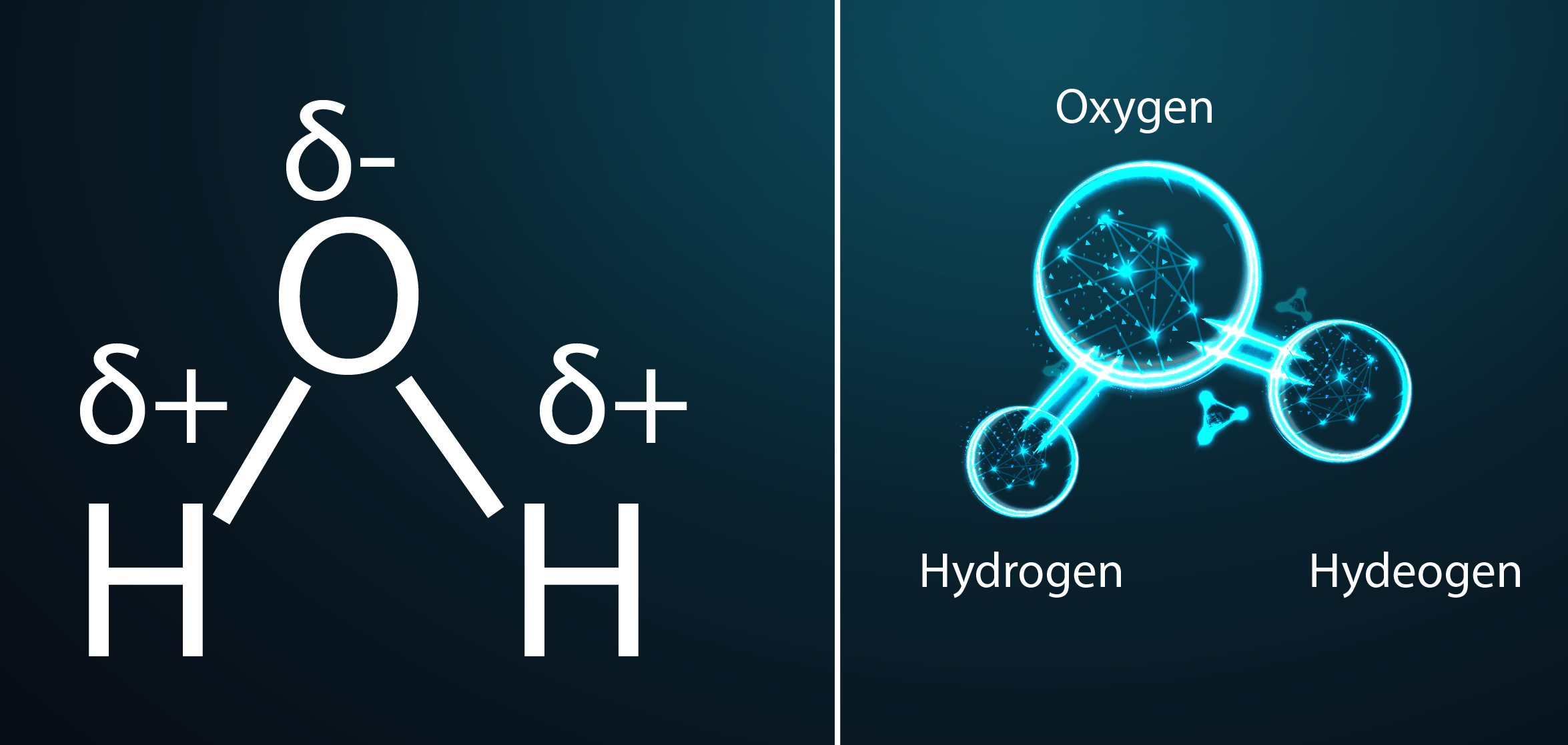
Water Structure and Polarity:
1 oxygen + 2 hydrogen make water with bent molecular geometry. Oxygen has 2 lone pairs, causing partial negative charge, while hydrogen has partial positive charge due to electronegativity difference. This charge difference creates polarity.
Important Properties of water :
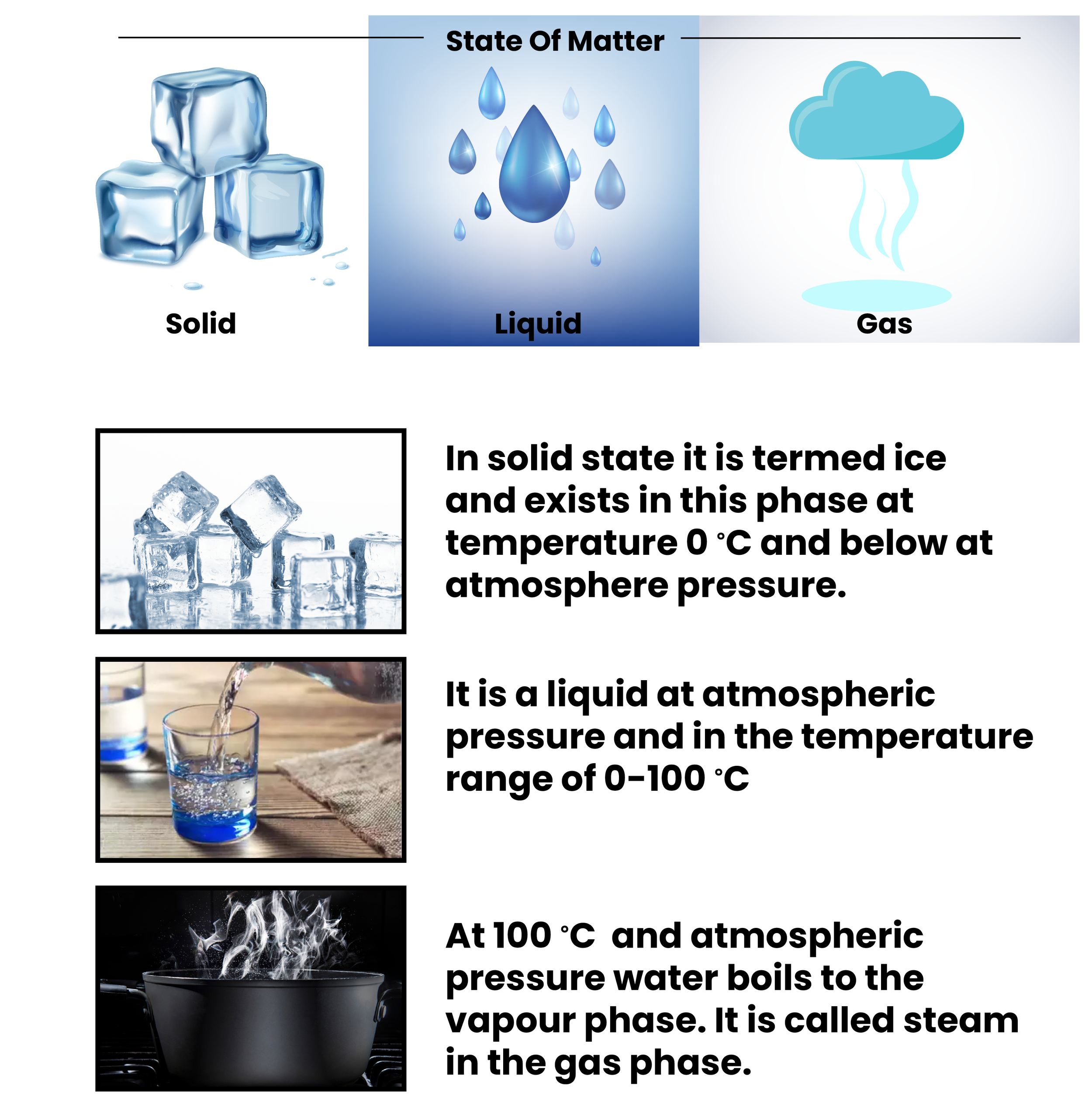
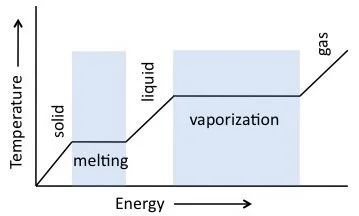
State of water: Water exists in all three states:
The following are the important physical properties with respect to water treatment:
Density : Pure water has a density of 1.0 kg/1 at 40 ℃ and atmospheric pressure. The density decreases at other temperature. The density of water increases with salinity. Sea water with a salinity of 3.5 g/l has a density of 1.028 kg/l at 0℃.
Thermal Properties :
The specific heat of water at 0℃ is 1 kcal/kg. The latent heat of fusion is 79 kcal/kg and the latent heat for vaporization is 539 kcal/kg at normal pressure and at 100℃.
Due to the large specific heat and latent heat of vaporization, water is used as a heat transfer fluid.
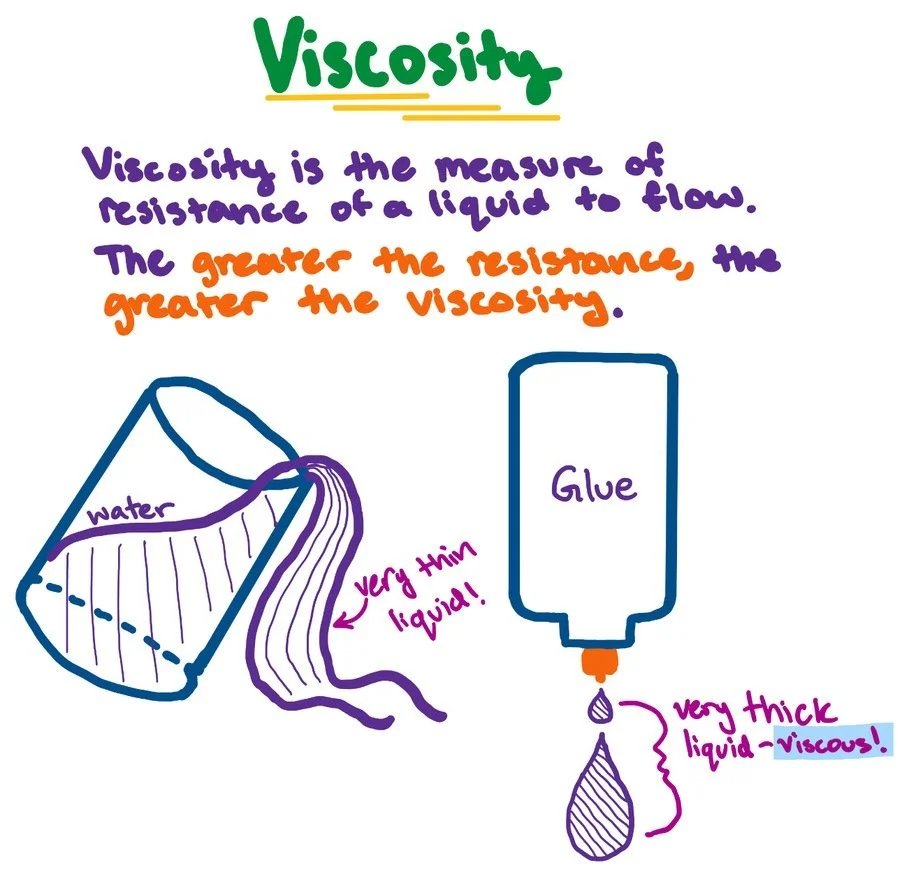
Viscosity :
This is the ability of a liquid to resist movements such as flow. It is the basic cause of head loss and therefore plays an important part in water treatment.
Viscosity of water reduces with increase in temperature.
Viscosity of water increases with higher content of dissolved salts. Sea water is therefore more viscous than river water.
Surface Tension :
This is a property peculiar to boundary surfaces of two phases. It is a tensile force which is exerted at the surface of the liquid and which tends to reduce the area of this surface to the greatest possible extent. Surface tension diminishes with a rise in temperature. The addition of dissolved salts generally increases surface tension.
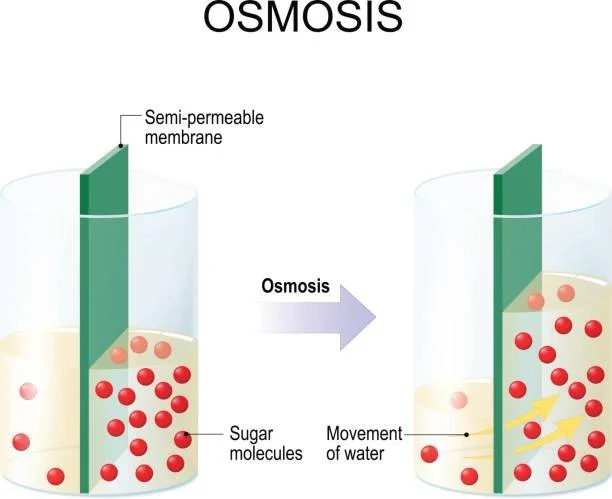
Osmotic Pressure :
The parameter is important for reverse osmosis sizing because it affects how well and efficiently the process works. П = ΔCRT
Where, П is the osmotic pressure in Pa.
ΔC is the difference in concentration across the membrane is mol/m3, R is the universal gas constant; R = 8.314 J/mole K. T is the temperature in K.
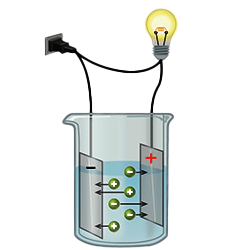
Electrical Conductivity
Pure water is indeed a fascinating substance that exhibits a subtle yet discernible level of conductivity. With a conductivity of just 0.037 micro siemens/cm, it might seem almost imperceptible to the Pure water is indeed a fascinating substance that exhibits a subtle yet discernible level of conductivity. With a conductivity of just 0.037 micro siemens/cm, it might seem almost imperceptible to the naked eye.
Compressibility : Compressibility is a result of pressure and temperature. The compressibility of water is so low that is often assumed to be incompressible. Low compressibility allows water in deep oceans with high pressure to only decrease by 1.8% in volume.
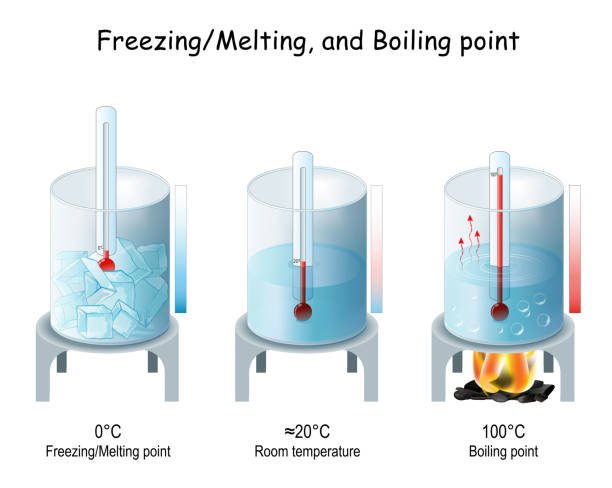
Boiling Point of Liquid : The boiling point is defined as the temperature at which the vapour pressure of the liquid is equal to the pressure surrounding the liquid, and thus the liquid changes to vapour. It is known to us that the boiling point of water is 100°C
Freezing Point of Liquid :
The freezing point is a crucial temperature parameter that signifies the transformation of a substance's state from liquid to solid. In the case of water, this noteworthy point is achieved when its liquid form gracefully transitions into the solid-state marvel that we commonly refer to as ice. It is worth noting that the freezing point of water is measured at the remarkably calm and crisp temperature of 0°C or 32°F.
References:
1. https://neoakruthi.com/blog/waste-water-treatment-methods.html
2. https://www.aquatechtrade.com/news/wastewater/wastewater-essential-guide
3. https://www.vedantu.com/chemistry/physical-and-chemical-properties-of-water
Explore Doshion's industrial water treatment resins for process transformation. Visit Doshion's Industrial Water Treatment Resins page : https://www.doshionpoly.com/ion-exchange-resin